About the AFFINITY clinical trial
AFFINITY. A clinical trial studying opicinumab in relapsing MS
- Overview
- About the investigational drug
- About AFFINITY
- Key AFFINITY features
- Clinical trial length
- Visits
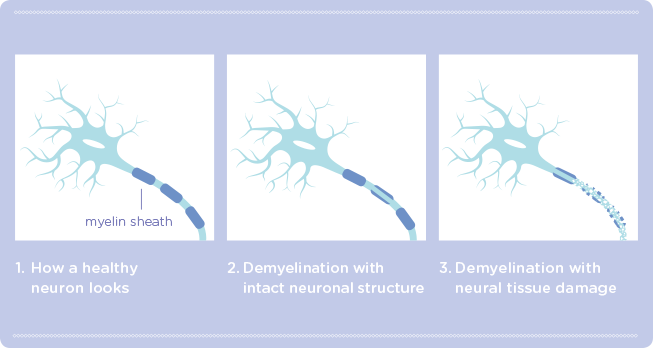
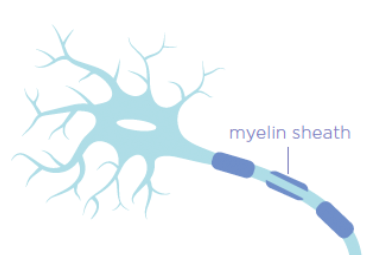
The immune system in people with multiple sclerosis (MS) attacks and damages the protective covering of the nerves in the brain and spinal cord called myelin. This makes it difficult for the nerves to function and to communicate with the rest of the body. Over time, these nerve fibers may be damaged as well and die, resulting in numbness, tiredness, problems with walking and thinking, as well as other symptoms.
How the investigational drug may work
- It is an antibody that targets a protein only expressed in the CNS (brain and spinal cord) called LINGO-1
- LINGO-1 inhibits the generation of new myelin and the repair of damaged myelin sheath (the protective covering around the nerves in your CNS)
- By blocking the activity of LINGO-1, opicinumab may potentially enhance the repair of myelin sheath, protect nerve fibers, and reverse deficits in neurological functions due to MS
About the AFFINITY clinical trial
AFFINITY is a clinical research trial testing opicinumab as a potential add-on therapy to disease-modifying therapies (DMTs) in relapsing multiple sclerosis (RMS). It is being evaluated to see if it may potentially repair tissue damaged by RMS, and to see if it may have a beneficial effect on disability.
Opicinumab was previously assessed in a Phase 2 clinical research trial called SYNERGY, however the results were not conclusive. Initial positive results were seen in a sub-set of patients who have relapsing MS that was diagnosed less than 20 years ago and met other specific criteria in their magnetic resonance imaging, or MRI, scans.
Based on the results of the SYNERGY clinical trial, we now have more information on the appropriate candidates for opicinumab. We are now looking for people with relapsing MS who match these criteria to take part in AFFINITY, our new Phase 2 clinical trial being conducted at approximately 150 research centers around the world.

Key features of AFFINITY
-
 All participants will continue to receive their current MS treatment* to control CNS inflammation and relapses
All participants will continue to receive their current MS treatment* to control CNS inflammation and relapses
-
 Half of all participants will receive opicinumab, while the other half will receive placebo
Half of all participants will receive opicinumab, while the other half will receive placebo
-
 Opicinumab is given by intravenous infusions
(a slow injection into the vein) every four weeks
Opicinumab is given by intravenous infusions
(a slow injection into the vein) every four weeks
-
 Total length of the clinical trial is approximately one and a half years
Total length of the clinical trial is approximately one and a half years
-
 A Phase 2 clinical trial
A Phase 2 clinical trial
Who we’re looking for
- Men and women
- 18-58 years old
- Less than 20 years from the first symptoms of MS
- Able to walk at least 50 meters with single-side walking aid or at least 120 meters with walking aids on both sides
- Have been receiving a Disease Modifying Treatment (DMT) for at least six months
- Be willing and able to undergo brain MRI scans every six months (maximum of four MRI scans) during the AFFINITY clinical trial (MRI results are critical to confirm your eligibility for the AFFINITY clinical trial, and are also used to monitor your health throughout the clinical trial)
Please note that other eligibility criteria apply. For those who meet the basic criteria and wish to participate, eligibility will be fully assessed at a ‘screening visit’.
*If you are taking one of the following DMTs you may be eligible for AFFINITY: Avonex®, Plegridy®, Betaferon®, Betaseron®, Rebif®, DMF, Tecfidera® or Tysabri®. You can continue to take your DMT throughout the clinical trial. Your neurologist may decide to switch you from one of these DMTs to another DMT during the clinical trial.
The following are trademarks of the respective companies listed: Betaferon®/Betaseron® (Bayer); Rebif® (EMD Serono, Inc.); Avonex®, Plegridy®, Tecfidera®, Tysabri® (Biogen).
Clinical trial length
How long will I be in the clinical trial for?
The total length of the AFFINITY clinical trial is up to 88 weeks (about one and a half years), including screening and follow-up, during which time you would have around 21 hospital visits. A brief outline of the clinical trial is shown in the chart below.
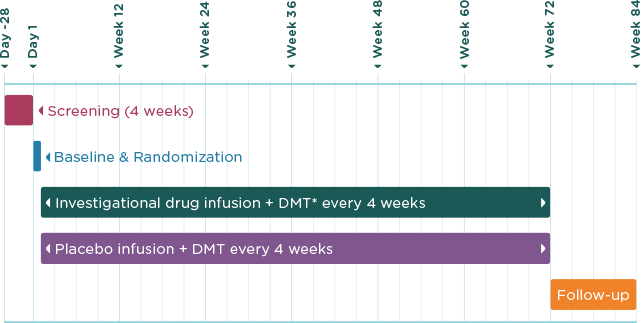
AFFINITY is divided into three periods:
Screening period (will last approximately one month)
The screening period is designed to check whether you are suitable for the clinical trial. You will need to visit the study center at least once during this four-week period.
Treatment period (will last approximately up to 18 months)
During the treatment period you will need to visit the study center every four weeks. At your visits, the study doctor will perform some tests to monitor your health and administer the study drug.
Follow-up period (will last approximately up to three months)
You will have a follow-up visit three months after your last dose of the study drug. At this visit, the study doctor will perform some tests to check your health.
*If you are taking one of the following DMTs you may be eligible for AFFINITY: Avonex®, Plegridy®, Betaferon®, Betaseron®, Rebif®, DMF, Tecfidera® or Tysabri®. You can continue to take your DMT throughout the clinical trial. Your neurologist may decide to switch you from one of these DMTs to another DMT during the clinical trial.
The following are trademarks of the respective companies listed: Betaferon®/Betaseron® (Bayer); Rebif® (EMD Serono, Inc.); Avonex®, Plegridy®, Tecfidera®, Tysabri® (Biogen).
Visits
If you decide to take part in the AFFINITY clinical trial, you will be asked to visit a study center about once a month so that we can give you the study drug and monitor your health. You will undergo a series of medical tests and procedures that may include:
-

Physical examination
-

Vital signs
-

Activities of thinking, learning and memory
-

Brain MRI
Magnetic Resonance Imaging -

Tests of vision, hand function and walking ability
-

ECG
An Electrocardiogram (ECG) is a test that measures and records the electrical impulses from a person's heart -

Blood and urine tests
Participants' blood and urine samples will be used to evaluate how the participant's body processes the investigational drug, in addition to other routine tests
Please note that not every assessment will be performed at every visit.
